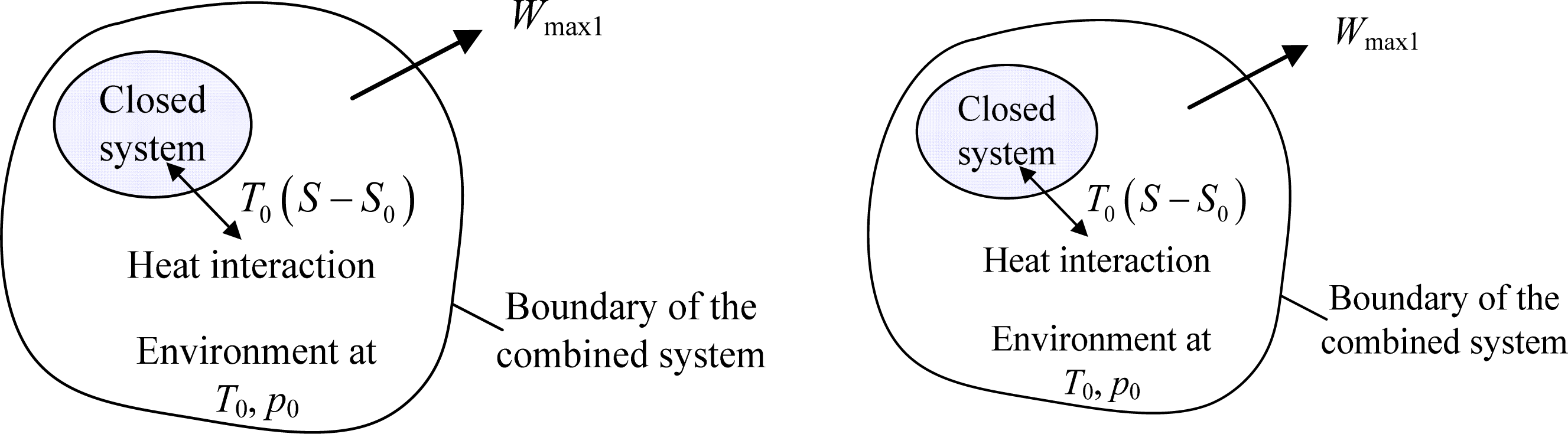
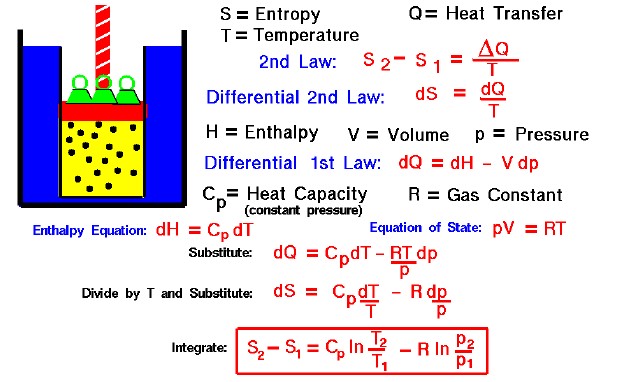
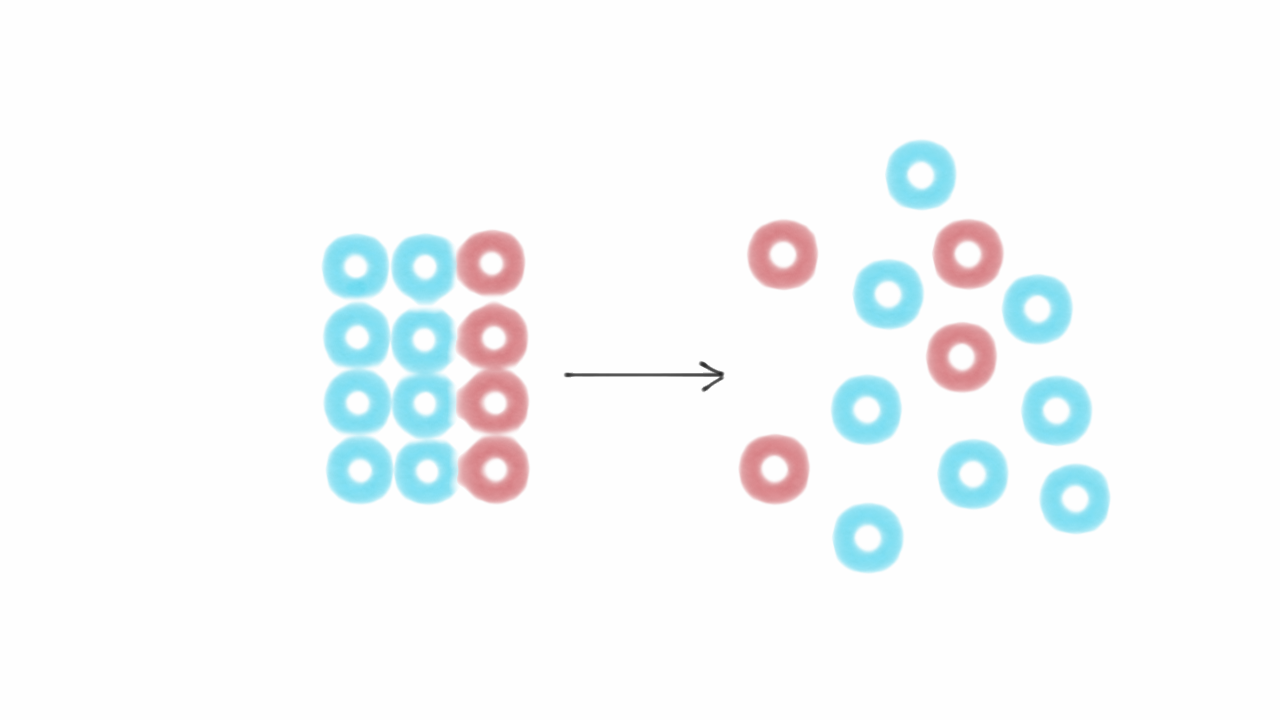
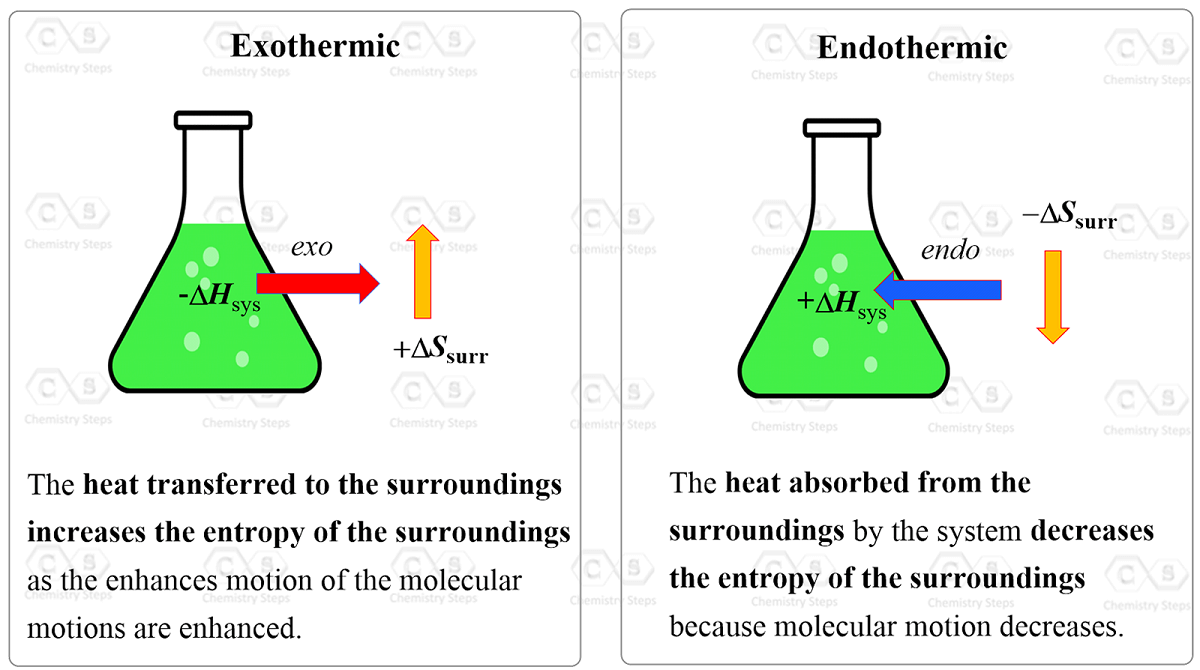
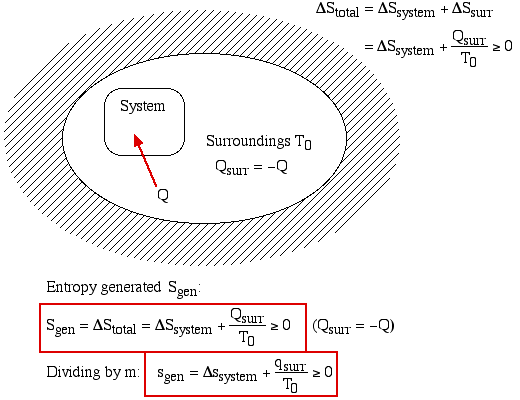
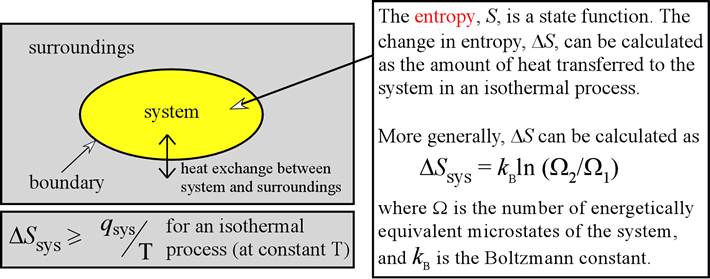
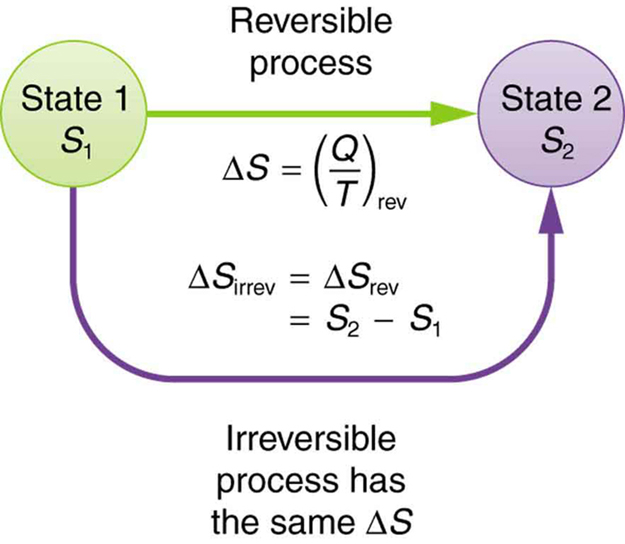
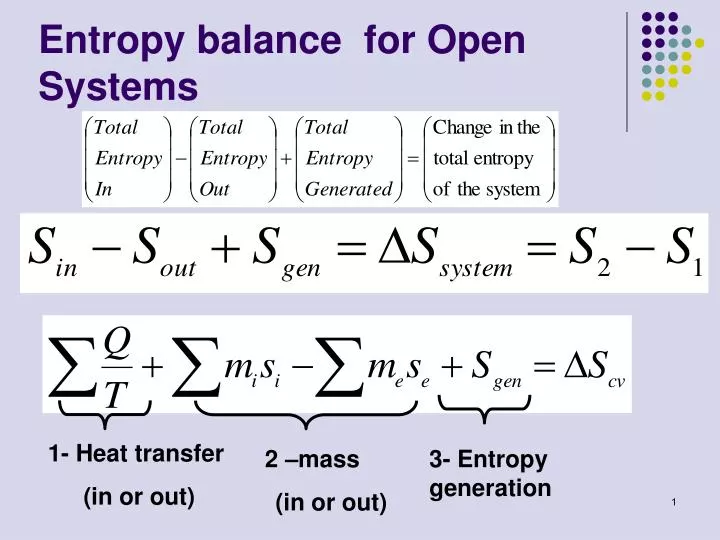
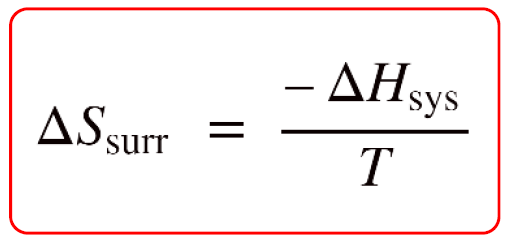What's the value of the sum of Entropy value of the system and surrounding together in reversible process?

6.6 The second law of thermodynamics for closed systems – Introduction to Engineering Thermodynamics
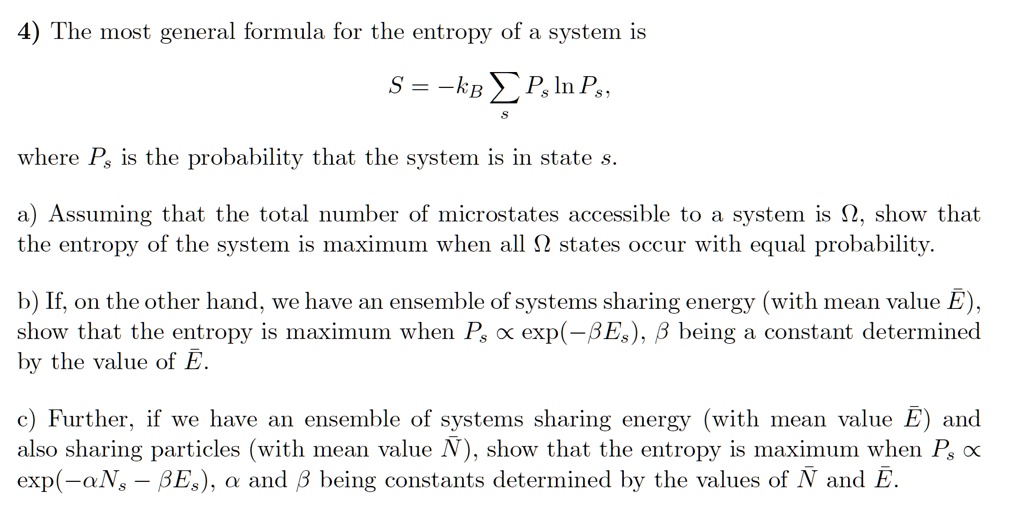
SOLVED: The most general formula for the entropy of a system is: S = -kB ln(Ps) In this formula, Ps represents the probability that the system is in a certain state. Assuming